近日,课题组硕士毕业生郭琛(现香港城市大学在读博士)的论文“Rational design and effective control of gold‐based bimetallic electrocatalyst for boosting CO2 reduction reaction: a first‐principles study”被 ChemSusChem(2020年中科院SCI期刊二区Top期刊,IF=7.962)接收!
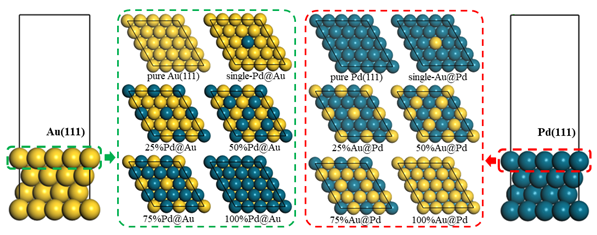
Electrochemical CO2 reduction reaction (CO2RR) is an effective strategy converting CO2 to value‐added products. Au is regarded as an efficient catalyst for electrochemical reduction of CO2 to CO, and the introduction of Pd can tune CO2RR properties due to its strong affinity to CO. Herein, Au‐Pd bimetallic electrocatalysts with different metal ratio were firstly investigated on CO2RR mechanism by using density functional theory. The Au monolayer over Pd substrate and single Pd atom on Au(111) were found to show better CO2RR selectivity against hydrogen evolution reaction (HER). Based on this, various single atom catalysts on Au(111) and core‐shell models with top Au monolayer were designed to study their CO2RR performance. The results indicated that Pt, Cu, and Rh substrates below Au monolayer could enhance the activity and selectivity for CO production than pure Au, in which the limiting potential reduced from ‐0.74 to ‐0.63, ‐0.69, and ‐0.71 V respectively. The single Pd embedded on Au(111) could adjust the adsorption strength, which provided an effective site to receive and further reduce CO to CH3OH and CH4 at a low limiting potential of ‐0.61 V, and also avoided catalyst poisoning due to the over‐strengthened CO adsorption caused by high Pd proportion on the surface. In addition, the adsorption energy of COOH was observed as a better CO2RR reactivity descriptor than the common CO adsorption when establishing scaling relationship, which could avoid the fitting error caused by intermediate physisorption of CO.
https://chemistry-europe.onlinelibrary.wiley.com/doi/10.1002/cssc.202100785